Haloperidol-Induced Catalepsy
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Drugs to treat schizophrenia and other psychological disorders induce well-described extrapyramidal side-effects. These side-effects have been exploited to create an animal model of catalepsy and cataleptic seizure.
For example, haloperidol administration to mice induces a well-described condition of immobility characterized by muscle rigidity and frozen posture. This condition in mice resembles similar human conditions in disorders such as Parkinson’s disease and schizophrenia. Indeed, this model has been developed to screen and evaluate test compounds for their ability to reverse these types of disease and drug-induced cataleptic seizure.
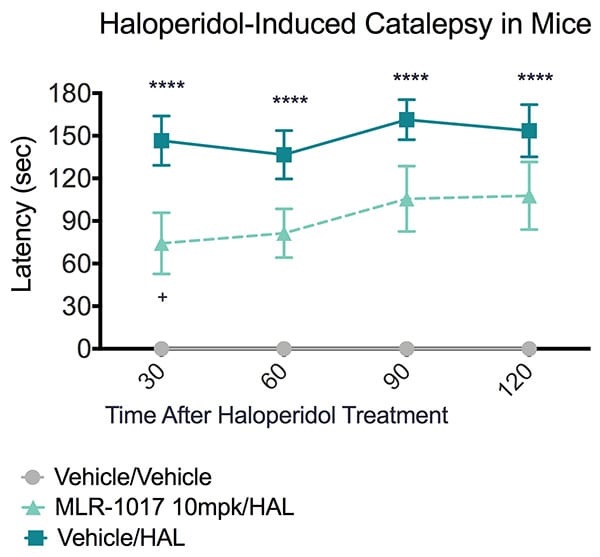
The study summarized in the figure below illustrates the effect of a dopamine re-uptake inhibitor in reducing catalepsy in this model.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
MLR-1017, a Dopamine Reputake Inhibitor, Reduced Cataleptic Seizure in Mice. Mice were pretreated with vehicle 30 minutes prior to study commencement. Mice were treated with either vehicle or haloperidol at study commencement (time=0) and evaluated for signs of catalepsy at 30, 60, 90 and 120 minutes after vehicle or Haloperidol treatment. Data are mean ± SEM; ****p<0.0001 +p<0.05 compared to vehicle/vehicle.
The bar test was utilized to measure Haloperidol-induced catalepsy. This test measures the ability of the animal to respond to drug-induced immobility (Kuschinsky and Hornykiewicz, 1972). The test measures the time required (latency) for the animal to remove its forelimbs from an elevated horizontal bar.
Animals were positioned so that their front paws are resting on a horizontal bar 6.5 cm high and the latency for the animal to remove both forepaws from the bar was recorded. A cut-off time of 180 seconds was imposed. In this study, haloperidol significantly increased the latency to remove both forelimbs from the horizontal bar compared to vehicle treated animals. MLR-1017 significantly improved performance of animals to respond after haloperidol treatment compared to vehicle-treated animals.
Reference
Kuschinsky K, Hornykiewicz O. (1974) Effects of morphine on striatal dopamine metabolism: possible mechanism of its opposite effect on locomotor activity in rats and mice. Eur J Pharmacol. 26(1):41-50