Pharmacokinetics
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Pharmacology is the study of the interactions between drugs and the living organism. The two divisions of pharmacology are pharmacokinetics and pharmacodynamics.
Pharmacokinetics (PK) refers to the movement of drugs through the body (adsorption, distribution, excretion and metabolism). Pharmacodynamics (PD) refers to the body’s biological response to drugs (behavior, receptor occupancy, qEEG and other biomarkers).
Melior customarily accompanies many of it animal model studies with PK analysis in order to get a more complete picture of the PK-PD relationship. Melior provides studies to address all aspects of PK and PD, including in vivo dosing via all routes, tissue and blood/plasma collection, bioanalysis, non-GLP noncompartmental analyses
These studies are useful for
- Drug exposure
- Pharmacokinetic modelling
- Prediction of dose requirements (PK/PD)
- Assess bioavailability/bioequivalence
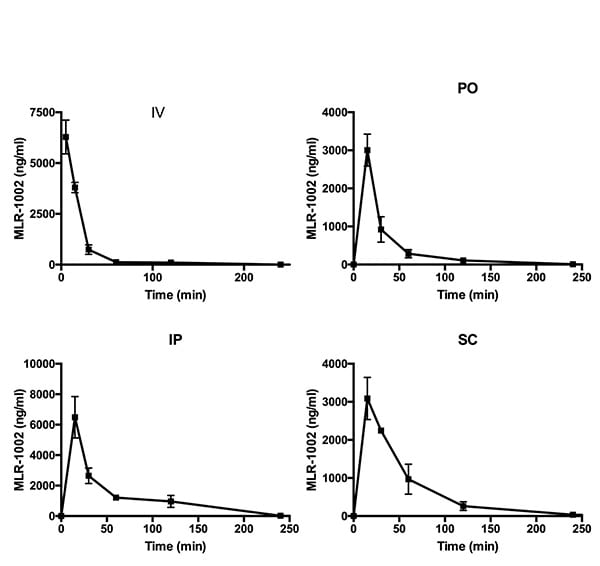
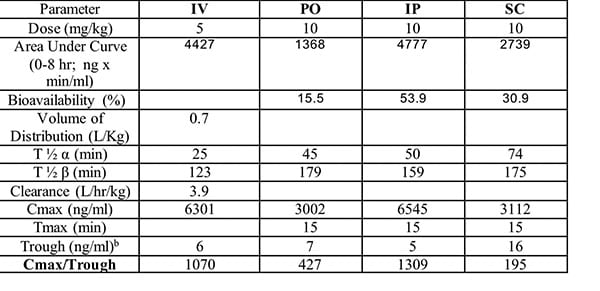
The figure below illustrates the summary output of a typical PK study in mice with a test article.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
Test article (MLR-1002) was administered by either IV, PO, IP, or SC route of administration as indicated. Compound was administered at 10mg/kg for PO, IP and SC routes of administration. Compound was administered at 5 mg/kg for PO route of administration. Blood was drawn 5, 15, 30, 60, 120, 240 and 480 min after drug administration. The following pharmacokinetic parameters were calculated: Volume of distribution (Vd), half-life (T ½ α :distribution phase; T ½ β:elimination phase), total drug exposure (area under the curve; AUC), clearance, oral bioavailability and Cmax (peak) and trough drug plasma levels.
The study served to show that best exposure was achieved by IP administration (highest AUC; 54% bioavailability). In addition for acute studies the PK characteristics of the compound suggest that evaluation of endpoints from 15-30 minutes after IP dosing may be optimal for assessing drug response. For chronic studies the PK characteristics of the compound suggest the BID (twice per day) may be preferred versus QD (once per day) due to the 2.6 hour half life.
Frequently Asked Questions
Yes. We work closely with a bioanalytical lab that has the ability to analyze blood and other tissue samples.
We think a PK analysis should be performed prior or in conjunction with any other study. Understanding how test articles are distributed and how long the compounds remains within the body is important for determining the route of administration and frequency of dosing.
Yes. The customized time points can be applied to the protocol.
Synonyms: PK, ADME, Pharmacodynamics