The HepG2 Xenograft Model for Liver Cancer
The Current HCC Treatment Landscape
Surgical resection and liver transplantation are the primary treatments for early-stage HCC, but many patients who present with advanced disease need systemic therapies. Advances in chemotherapy, targeted therapy, and immunotherapy have improved outcomes, but more effective treatments are urgently needed.
Drug discovery in HCC also faces several challenges, including the tumor’s genetic heterogeneity, the immunosuppressive microenvironment, and the development of drug resistance. These factors complicate the identification and validation of new therapeutic targets and hinder the effectiveness of existing treatments. Additionally, the liver’s unique physiology and its role in drug metabolism make it challenging to develop therapies that are both effective and safe.
Melior’s HepG2 xenograft model addresses these challenges by providing a reliable platform for preclinical testing. It mimics the human liver tumor microenvironment, enabling the evaluation of drug efficacy and toxicity in conditions resembling clinical settings and accelerating the development of effective HCC treatments.
Advantages of the HepG2 xenograft model
- Scale your research with highly reproducible tumor growth.
- Utilize natural liver enzyme activity for realistic drug metabolism.
- Improve data reliability with consistent tumor model performance.
- Reduce study time with short lead timelines for tumor establishment in mice.
Track tumor progression without sacrifice using the
HepG2-Luc2 xenograft model
As a derivative of the HepG2 cell line, the HepG2-Luc2 xenograft model retains the same stability, reliability, and decades-long track record but has been engineered to express the luciferase 2 (Luc2) gene. Used often in molecular biology for live tracking, Luc2 is a bioluminescent reporter that allows for noninvasively monitoring cells in real time using IVIS and other bioluminescence imaging techniques.
Using the HepG2-Luc2 xenograft model, monitor tumor growth, metastasis, and response to treatment at multiple time points without sacrificing the animal.
Investigate liver cancer in greater detail with our custom services
Unlock the full potential of your liver cancer research with our HepG2 xenograft model. Our specialized services offer precise insights into tumor biology and therapeutic efficacy.
Contact us for more information on our additional and individualized services, including IVIS imaging, PK studies, and metabolic analyses.
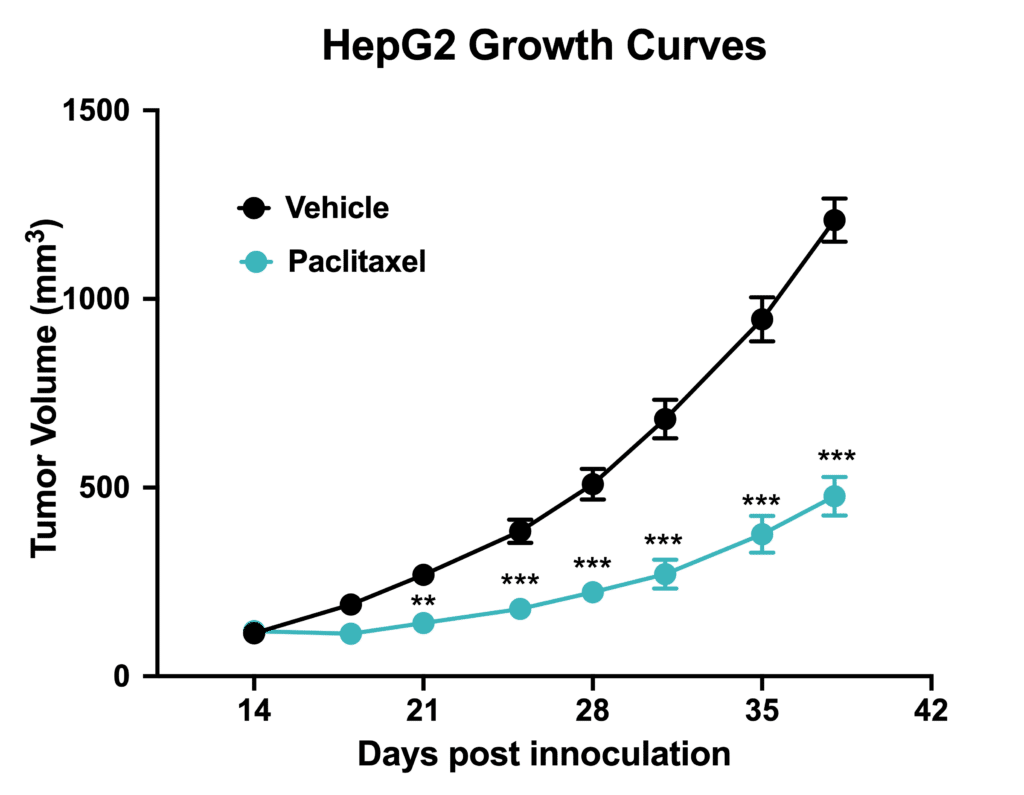
Chemotherapy validation in human hepatic cancer HepG2 xenograft model. 5 x106 HepG2 cells were subcutaneously injected into the rear flank of nude mice. Once the tumor size reached ~100mm3, mice were randomized into vehicle group (n=6,treated with normal saline) and paclitaxel group (n=5, 20 mg/kg, IP weekly).Data are mean ± SEM. **p<0.01, ***p<0.001.
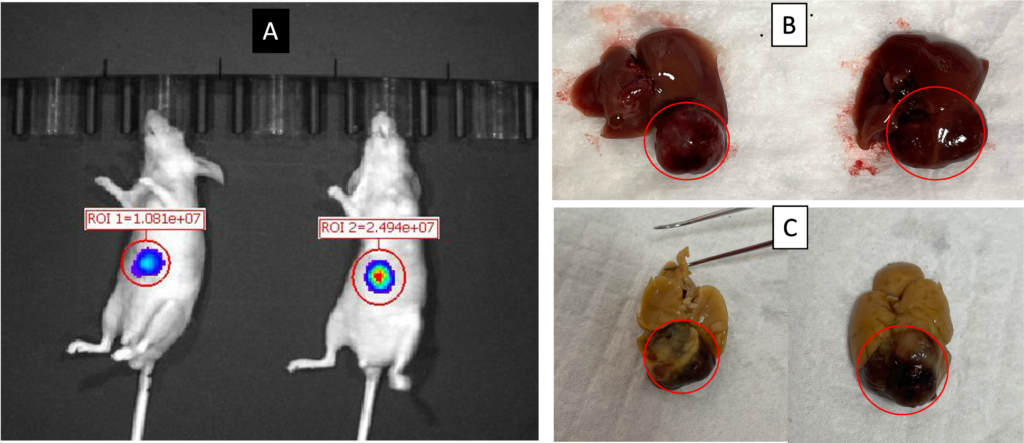
The HepG2 orthotopic liver tumor model was generated by inoculating 1 × 106 HepG2 cells into the left liver lobe of nude mice through laparotomy under isoflurane anesthesia. IVIS-BLI was performed at week 4 (A). The mice were euthanized at week 8 weeks and the liver were excised (B) and formalin fixed (C). Red circle indicated HepG2 tumor.
Did you know the HepG2 cell line mimics human liver cancer metabolism?
Our HepG2 xenograft model not only supports reliable tumor growth but also replicates human liver cancer metabolism, providing deeper insights into drug efficacy. This unique capability ensures more accurate preclinical results. With customized study designs and short lead times, accelerate your HCC research effectively.
Publications
Frequently Asked Questions
The HepG2 xenograft model offers highly reproducible tumor growth, natural liver enzyme activity for realistic drug metabolism, consistent tumor model performance, and short lead timelines for tumor establishment in mice. Being an HCC mouse model, it also represents the majority of liver cancers, as over 75% of liver cancers are HCC.
These advantages help streamline clinical development and improve the accuracy of preclinical drug efficacy predictions.
The HepG2 xenograft model mimics human liver cancer metabolism by replicating key metabolic pathways and enzyme activities characteristic of HCC. HepG2 cells express liver-specific enzymes and proteins such as cytochrome P450 enzymes, albumin, and alpha-fetoprotein, which are crucial for drug metabolism and liver function. Additionally, HepG2 cells maintain essential liver functions like gluconeogenesis, glycogenolysis, and lipid metabolism. They also exhibit the Warburg effect, where cancer cells prefer glycolysis over oxidative phosphorylation, leading to increased glucose uptake and lactate production, mimicking metabolic shifts observed in HCC.
This capability ensures that the HCC mouse model accurately reflects the metabolic environment of human liver cancer, leading to more accurate preclinical results and better predictions of clinical outcomes.
The HepG2-Luc2 xenograft model is a derivative of the HepG2 cell line engineered to express the luciferase 2 (Luc2) gene. This bioluminescent reporter allows for noninvasive real-time monitoring of tumor growth, metastasis, and treatment response using IVIS and other bioluminescence imaging techniques, providing precise and dynamic tumor insights without sacrificing the animal.
Citations
- Serraino, D., Fratino, L., Piselli, P. (2023). Epidemiological Aspects of Hepatocellular Carcinoma. In: Ettorre, G.M. (eds) Hepatocellular Carcinoma. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-09371-5_1.
- Tolou-Ghamari Zahra. (2024). Review of Hepatocellular Carcinoma and Liver Disease Prevalence, New Emirates Medical Journal; Volume 5, e02506882271605. DOI: 10.2174/0102506882271605231211102803. Available at: https://www.benthamscience.com/article/139873.
- Pinto-Marques, H; Cardoso, J; Silva, S; Neto, JL; Gonçalves-Reis, M; Proença, D; Mesquita, M; Manso, A; Carapeta, S; Sobral, M; Figueiredo, A; Rodrigues, C; Milheiro, A; Carvalho, A; Perdigoto, R; Barroso, E; Pereira-Leal, J. (2022). A Gene Expression Signature to Select Hepatocellular Carcinoma Patients for Liver Transplantation. Annals of Surgery 276(5):p 868-874. DOI: 10.1097/SLA.0000000000005637 Available at: https://pubmed.ncbi.nlm.nih.gov/35916378/.
- Aden, D., Fogel, A., Plotkin, S. et al. (1979). Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282, 615–616. https://doi.org/10.1038/282615a0.
- Sheng-Long Ye, Tadatoshi Takayama, Jeff Geschwind, Jorge A. Marrero, Jean-Pierre Bronowicki, Current Approaches to the Treatment of Early Hepatocellular Carcinoma, The Oncologist, Volume 15, Issue S4, November 2010, Pages 34–41, https://doi.org/10.1634/theoncologist.2010-S4-34.
- Kinsey, E.; Lee, H.M. (2024). Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers, 16, 666. https://doi.org/10.3390/cancers16030666.