The LNCaP Xenograft Model for Prostate Cancer
Mimicking the Natural Progression of Prostate Cancer
LNCaP cells were originally isolated in 1977 from metastasis in the left supraclavicular lymph node of a 50-year-old Caucasian male. Being metastatic, slow-growing, androgen-sensitive prostate cancer cells, ideal for modeling the typical progression of prostate cancer for therapeutic development.
Although 2D cell culture models are good at providing foundational insights, the LNCaP xenograft mouse model offers an in vivo environment that better reflects clinical conditions. This ensures comprehensive preclinical testing in a model that mimics the majority of prostate cancers.
Advantages of the LNCaP Xenograft Model
- Achieve relevant insights with androgen-sensitive human prostate cancer cells
- Reduce lead timelines with 4-6 week tumor establishment in mice to shorten study time
- Adapt to research challenges with flexible delivery, including subcutaneous and orthotopic xenografts
- Rely on a dependable cell line elevated to an innovative 3D culture system
Personalize your prostate cancer research with our custom services
Elevate your prostate cancer research with our LNCaP xenograft model, ideal for studying androgen receptor signaling and therapeutic efficacy in vivo.
Contact us for more information on our bespoke services, including IVIS imaging, PK studies, and tumor biomarker analyses.
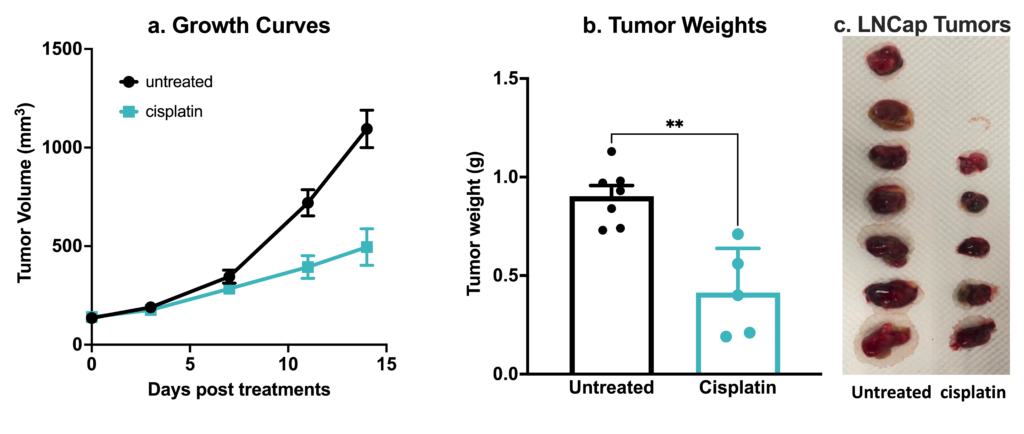
Human prostate cancer LNCaP xenograft model. 2.5×106 LNCaP cells were subcutaneously injected into the rear flank of nude mice. Once the tumor size reached ~150mm3 (Day 30), mice were randomized into untreated control and cisplatin group (4 mg/kg, IP BIW). Tumor volume was monitored 2x weekly using calipers (A). At the end of the study (Day 44), animals were sacrificed, tumors excised and weighted (B,C). Data are mean ± SEM; n=5 / group; ** p<0.01, by Welch’s t-test
Did you know we offer comprehensive sample collection with our LNCaP xenograft model?
We provide collection services for blood and tissue samples for PK, flow cytometry, ELISA, H&E, HIF/HIC, and more. These samples provide critical data on tumor progression, treatment response, and biomarker analysis, enhancing the depth of your research.
Publications
Frequently Asked Questions
The LNCaP cell line is androgen-sensitive, making it ideal for modeling early-stage prostate cancer and androgen receptor signaling. PC3 and DU145 are both androgen-independent, representing more aggressive, late-stage prostate cancer. LNCaP cells are best for studying the transition from androgen dependence to independence, while the cell lines PC3 and DU145 are valuable for research on advanced, hormone-refractory prostate cancer.
The LNCaP xenograft model is ideal for use in various study types, including:
- Drug efficacy testing
- Androgen receptor signaling
- Tumor biomarker analyses
- Pharmacokinetics (PK) studies
The model also supports personalized services like IVIS imaging for monitoring tumor growth and metastasis.
The LNCaP xenograft model can be ready for treatment in 4-6 weeks following cell inoculation, and the treatment window can be 8-10 weeks.
Citations
- Heinlein, C. A., Chang, C. Androgen Receptor in Prostate Cancer. EndocrineReviews, 25(2), 276–308. https://doi.org/10.1210/er.2002-0032
- Horoszewicz, J. S., Leong, S. S., Kawinski, E., Karr, J. P., Rosenthal, H., Chu, T. M., Mirand, E. A., & Murphy, G. P. (1983). LNCaP model of human prostatic carcinoma. Cancer research, 43(4), 1809–1818.
- James, N. D., Tannock, I., N’Dow, J., Feng, F., Gillessen, S., Ali, S. A., Trujillo, B., Al-Lazikani, B., Attard, G., Bray, F., Compérat, E., Eeles, R., Fatiregun, O., Grist, E., Halabi, S., Haran, Á., Herchenhorn, D., Hofman, M. S., Jalloh, M., Loeb, S., … Xie, L. P. (2024). The Lancet Commission on prostate cancer: planning for the surge in cases. Lancet (London, England), 403(10437), 1683–1722. https://doi.org/10.1016/S0140-6736(24)00651-2
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660