MOG-Induced EAE Model of Multiple Sclerosis
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
Experimental autoimmune encephalomyelitis (EAE) is the most commonly used animal model of multiple sclerosis (MS), as it shares clinical and pathophysiological features to human disease. EAE is classically characterized by CNS inflammation, demyelination, and paralysis. The myelin oligodendrocyte glycoprotein (MOG35-55) -induced EAE model is an active acute form of the disease as opposed to a relapse/remitting form of the disease in the PLP-induced EAE model.
In this model, EAE is induced in female C57Bl/6 mice by immunization with MOG35-55 in complete Freund’s adjuvant (CFA) emulsion followed by a two-time administration of pertussis toxin (PTX). The MOG35-55 peptide initiates expansion and differentiation of MOG-specific autoimmune T cells. PTX enhances disease development by modulating immunological response and facilitating the movement of autoimmune T cells into the CNS.
In this study, we assessed the progression of locomotor dysfunction in mice using with or without treatment with the immune modulator FTY-720 (fingolimod; GilenyaÒ). FTY-720 treatment was administered therapeutically beginning Day 14 after MOG35-55 immunization. Body weights and clinical scores were measured daily Days 7-21.
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
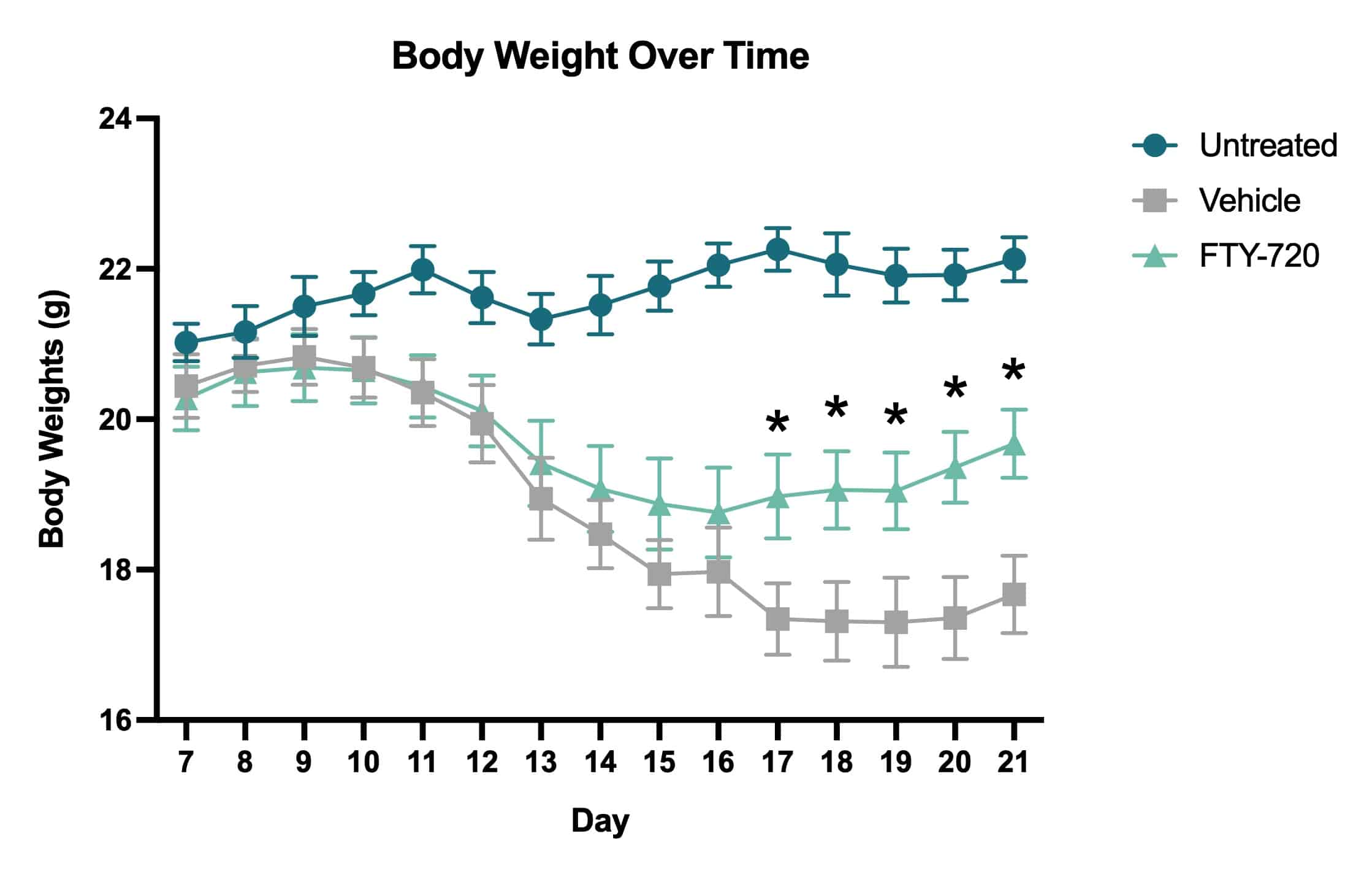
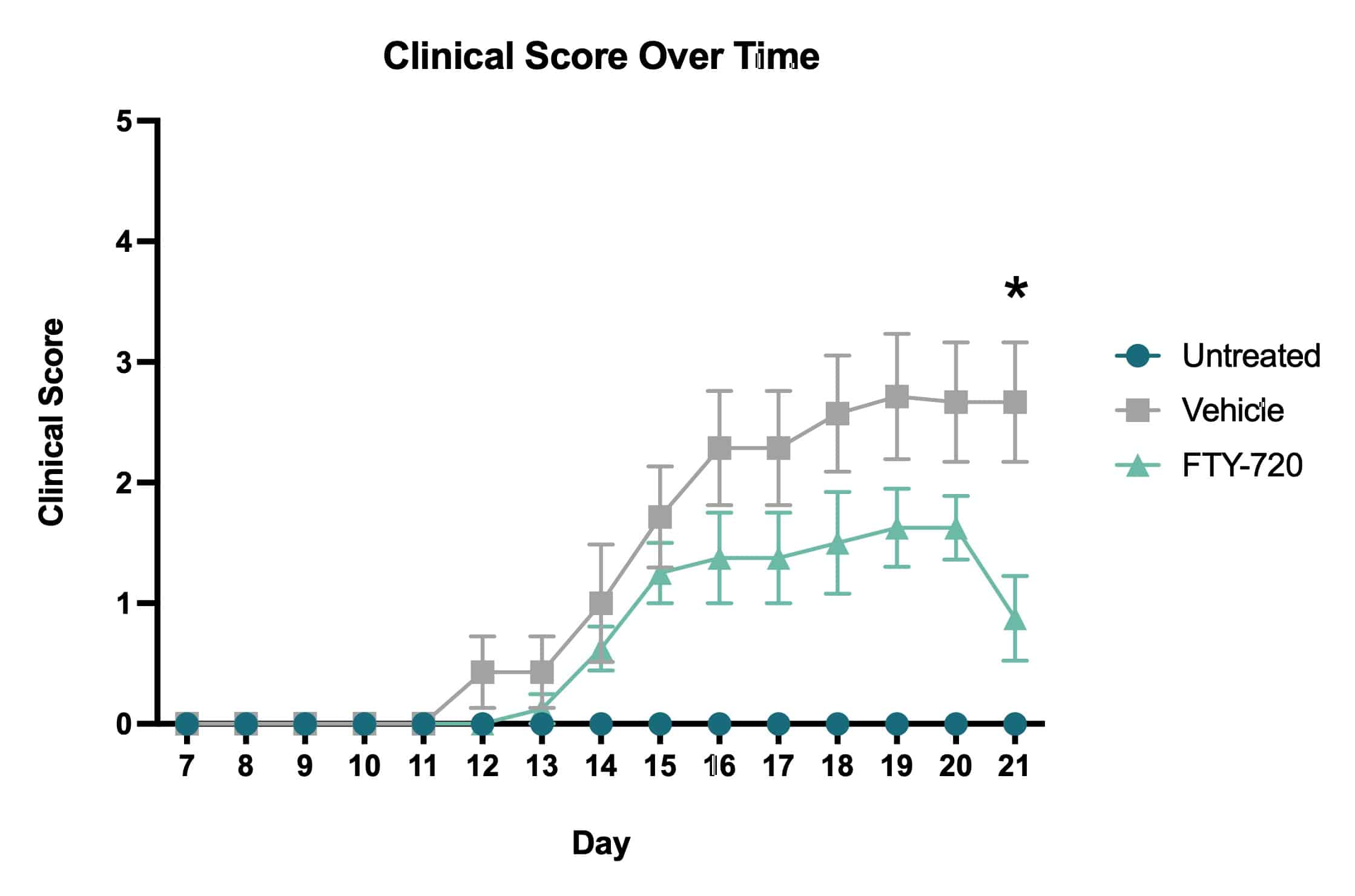
Average clinical scores over time. Clinical scores were assessed daily beginning on Day 7 and ending on Day 21. Based on the clinical score system, vehicle treated mice showed an increase in impairment severity throughout the course of the study. The FTY-720 treated mice showed a decrease in impairment following drug administration that became statistically significant when compared to vehicle treated mice on Day 21 (*p<0.05).
Clinical Scoring: Mice were randomized according to clinical score on Day 7 of the study. Clinical scoring was completed so that each mouse received one score per day. Clinical scores were evaluated using the following scale:
| Clinical Score | Symptoms |
|---|---|
| 0 | No clinical signs; normal activity |
| 1 | Limp tail or hind limb weakness, but not both |
| 2 | Limp tail and hind limb weakness |
| 3 | Partial hind limb paralysis |
| 4 | Complete hind limb paralysis |
| 5 | Moribund state; death by EAE |
The MOG35-55-induced EAE Model is performed in mice. Typical study duration is about 21 days.
